Offer
Provide additional details about the offer you're running.

Written by Dr Sarah Ballantyne, The Paleo Mom.
It’s no secret that I’m a huge broth fan! Between its helpful mineral content and abundance of important amino acids (especially proline and glycine), it’s definitely meets the criteria for a healing and nourishing food to include in our diets.
But, every once in a while, an article makes the rounds claiming that bone broth can actually be dangerous—and that some of us should either steer clear of it entirely, or only consume broths that have been cooked for short periods of time (in contrast to the long-simmered broths that have higher nutritional content).
So, what could possibly be wrong with this nutrient-dense, traditional food? The arguments against broth usually focus on two things:
Yikes! Both of those sound pretty bad, and plenty of articles offer seemingly scientific explanations for why they’re legitimate concerns. But, what does the evidence really say? Should we be wary of broth, or embrace it as a health-promoting food? Let’s look at the science to find out the truth!
In the “bone broth is harmful” discussion, a few different words get thrown around that start with the letters “glutam”—but they’re not all the same thing! So before we get started, let’s look at some definitions to help clear things up:
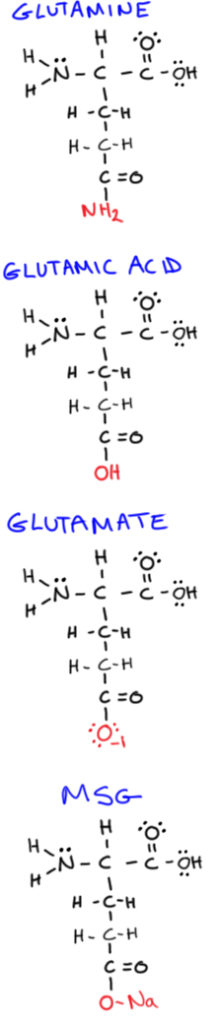 Glutamine
Glutamine Glutamine is a non-essential amino acid (the most abundant one in the human body!), and is one of the few amino acids that can directly cross the blood-brain barrier.
Sometimes used interchangeably with glutamate, this is a non-essential amino acid closely related (in a molecular structure sense) to glutamine.
[Aside: Glutamine is typically shorted as gln or Q in amino acid sequences of proteins, whereas glutamic acid is shortened as glu or E. However, as you can probably guess by their incredibly closely related molecular structures, its sometimes not possible to distinguish whether it’s glutamine or glutamic acid in a protein structure, in which case its shortened as glx or Z.]
This is an excitatory neurotransmitter needed for normal learning and memory. But, excessive levels in the brain have been associated with neurological diseases, stroke, and ALS. It forms when glutamic acid is bound to an anion or salt.
Also called MSG, this is the sodium salt of glutamic acid (containing only sodium and glutamate), and is commonly added to food as a flavor enhancer.
A form of glutamate that’s bound to other amino acids, and is generally digested and absorbed slowly.
A form of glutamate that isn’t bound to other amino acids and may be absorbed more rapidly by the body (MSG is one example). Free glutamate is what gives certain foods an “umami” taste, like soy sauce, tomatoes, and Parmesan cheese.
The theory about free glutamates, which mostly comes from Russell Baylock (author of “Excitotoxins: The Taste That Kills), proposes is that exposure to high levels of free glutamates causes them to reach the bloodstream too quickly, overexcite neurons, and potentially cause damage—resulting in symptoms like insomnia, migraines, anxiety, restlessness, irritability, or the aggravation of existing neurological diseases. (On the other hand, some research has shown free glutamate to play a beneficial role in regulating gastrointestinal functions, and could even help treat functional dyspepsia, chronic atrophic gastritis, maldigestion, and insufficient digestion among the elderly.)
There’s sufficient evidence suggesting that high glutamate levels in the brain can be harmful. It’s been associated with a number of neurological conditions, including autism and multiple sclerosis, and may also contribute to migraine headaches. But, this is much different than saying the levels of glutamate in bone broth can directly pass through the blood-brain barrier (a layer of cells surrounding most of the brain) and cause harm!
Under normal circumstances, our bodies are able to tightly regulate the blood-brain barrier and prevent excess glutamate from getting through (since specific receptors control the amount that can enter). Plus, most of the glutamate we eat gets metabolized in the gastrointestinal tract by intestinal cells, or converted by the liver. So, for most people, dietary glutamate isn’t going to have a significant effect on the levels in our brains.
For neurological conditions, the idea of “free glutamate sensitivity” is part of the rationale for avoiding high-glutamate foods. In this case, the problem isn’t necessarily dietary intake of free glutamate, but an issue of either genetic problems with glutamate transporters, or a “leaky” blood-brain barrier that allows too much glutamate to enter (leading to the conclusion that reducing dietary glutamate can work therapeutically).
It makes sense in theory! But, the problem is, free glutamate sensitivity is not well understood. Most of the information we have is speculation and anecdote, and clinical trials on people who claim to be sensitive to free glutamates (like MSG) have been mixed. In clinical trials, only extremely high levels of free glutamate (such as 5 grams, typically in the form of MSG) have been shown to induce adverse symptoms in humans—and even then, it’s only in a small fraction of the population. We don’t currently have enough data to understand the phenomena of glutamate sensitivity and how different nutrient-dense foods (not just high amounts of food additives) are involved!
When it comes to bone broth, the potential concern is that it has high levels of glutamate—especiallyfree glutamate. The logic goes that cooking bones for long periods of time breaks down naturally occurring amino acids and increases the concentration of free glutamate, causing some of the same problems associated with MSG and other unbound glutamate sources. And, in theory, adding vinegar to broth (a common technique believed to help break the bones down faster) supposedly raises the free glutamate content even higher by hydrolyzing the existing proteins!
There are a few problems with this idea. First, analyses of the amino acid content of broth (such as a recent one performed by Covance Laboratories in Wisconsin, using long-cooked bones from organically raised chickens) found that 8 oz. of broth contained about 1013 mg of glutamic acid. That might sound like a lot, but it’s actually less than many other foods! For example, a filet of salmon contains 12,940 mg of glutamic acid, one roasted chicken breast contains 8620 mg, 1 cup of boiled lobster contains 5060 mg, 3 oz. of turkey breast contains 3860 mg, 3 oz. of broiled ground beef contains 3280 mg, half a cup of unsalted tomato paste contains 1930 mg, and an ounce of walnuts contains 1420 mg. Some articles warning that bone broth is dangerous (like “The Dark Side of Bone Broth”) have named glutamic acid in general as a problem with long-cooked broths. But clearly, if we should be suspicious of broth just on the basis of its total glutamic acid content, there are plenty of other Paleo-friendly foods we should be even more concerned about!
More importantly, we have no idea whether the glutamic acid measured in broth is bound (making it theoretically less harmful) or free (making it behave similarly to MSG). Existing studies have only reported total glutamic acid content, without separating bound and unbound sources. Even people promoting the idea that bone broth could be harmful due to its free glutamate content, such asbiochemist Katherine Reid, acknowledge that it’s only speculation that glutamate gets freed up in broth and that we don’t know how much is actually released.
So, right now, the idea that long-time cooking and acidity from vinegar enhance the creation of free glutamate makes some sense in theory, but we have no way of confirming what level of free glutamates it might actually contain. Plus, it’s highly unlikely that even very long periods of cooking bones would convert all of the existing glutamic acid into free glutamate.
By contrast, we do know the free glutamate content of some other foods. Per 100 g, Roquefort cheese has 1280 mg, parmesan cheese has 1200 mg, walnuts have 658 mg, fresh tomato juice has 260 mg, peas have 200 mg, red grapes have 184 mg, broccoli has 176 mg, ripe tomatoes have 140 mg, potatoes have 102 mg, and human breast milk has 18 mg. Even if cooking bones for extended periods does release some free glutamate, the levels probably don’t exceed those of many of these foods (especially given the lower levels of glutamic acid as a starting place). So again, if we’re going to single out free glutamate as a health danger, a lot of other nutritious foods will have to get blacklisted too!
Another common belief about bone broth is that adding vinegar to the broth water causes protein to hydrolyze, releasing even more free glutamate from the bones. (As we saw in Why Broth is Awesome, there’s also a belief that adding vinegar helps pull out significantly more calcium, but this turned out to be a myth!)
One way chemists hydrolyze protein into free amino acids is by boiling the protein in a strong acid (usually 6N hydrochloric acid or 6N sulfuric acid) for 24 hours. By the end of that period, the free amino acids and peptides have been liberated from the original protein source. So, it’s certainly true that long-term exposure to high acidity and heat can help break down proteins and lead to the formation of free amino acids, including free glutamate.
But, that’s only part of the story! When making bone broth, we aren’t boiling bones in high concentrations of a strong acid like they do in a lab. Not only is vinegar only 5% acetic acid (a very weak acid compared to hydrochloric and sulfuric acid), but the vinegar then gets diluted in large amounts of water during cooking (typically 1 Tbsp per gallon or two of water). As a result, the amount of acid that ends up in the broth is extremely small! Likewise, while strong acids are used for fully hydrolyzing proteins into individual amino acids, weak acids are mostly capable of cleaving proteins at aspartic acid residues—which isn’t the same as releasing free amino acids like glutamates.
So, even though we don’t have any exact data on how the addition of vinegar influences protein breakdown from bones (and the level of free glutamates that end up in the broth), we can use logic and basic math to conclude that the effect is probably trivial. If it takes 24 hours of boiling protein in a strong, highly concentrated acid to release all the free amino acids, then using liquid that’s only 0.01% weak acid (the amount from mixing a tablespoon of 5% acetic acid with a whole gallon of water) probably isn’t going to do very much!
Bottom line: we really shouldn’t worry about a few splashes of vinegar dramatically increasing the free glutamates in our broth. Chances are, the effects are negligible!
Another common argument against bone broth involves heavy metals. Because heavy metals bind to apatite (the primary mineral component of bones and teeth), there’s some concern that bones sequester heavy metals and contain dangerously high levels (which, in turn, could seep into broth!). This is especially true for bones from animals that are exposed to heavy metals in their environment or feed.
This fear seemed to be confirmed by a 2013 analysis of broth, which found that organic chicken bones did indeed release lead into the water they were boiled in (concentrations of 9.5 ug/L and 7.01 ug/L, respectively, for broth made from chicken skin and cartilage versus broth made just from chicken bones). That sounds pretty bad, right?
But, here’s the catch! Those numbers mean nothing without context. It turns out, the amount of lead in the bone broth was still well below the Environmental Protection Agency’s safe upper limit for lead in drinking water (15 ug/L) and wouldn’t feasibly be high enough to cause harm. And, a number of other nutrients abundant on a real-food Paleo diet can help protect against lead toxicity: vitamin B, vitamin C, vitamin D, calcium, and iron. That makes lead exposure from broth an even less likely problem in the context of a healthy diet.
So, should we be worried? Based on the evidence, there’s really no reason to fear heavy metal poisoning from bone broth! But, if we want to make extra sure our broth is free from contaminants, the best bet is to use bones from 100% pastured animals raised on small-scale farms away from industrialized areas (to minimize their environmental exposure to heavy metals), as well as making broth at home with filtered water (instead of using store-bought brands).
 Right now, we don’t have enough research to confirm that bone broth could be harmful due to its free glutamate content. Of course, anyone who experiences negative reactions after eating it should steer clear (regardless of what the scientific explanation is—other factors like histamine or food intolerance to one of the ingredients could also be at play). But for the rest of us, avoiding broth on the basis of theoretical, unproven dangers would mean missing out on a truly amazing, nutritious food.
Right now, we don’t have enough research to confirm that bone broth could be harmful due to its free glutamate content. Of course, anyone who experiences negative reactions after eating it should steer clear (regardless of what the scientific explanation is—other factors like histamine or food intolerance to one of the ingredients could also be at play). But for the rest of us, avoiding broth on the basis of theoretical, unproven dangers would mean missing out on a truly amazing, nutritious food.
“Basic Information about Lead in Drinking Water.” United States Environmental Protection Agency. Accessed April 19, 2016.
Giacometti T. “Free and bound glutamate in natural products.” Glutamic acid: advances in biochemistry and physiology. New York: Raven Press, 1979:25–34.
Hands ES. “Nutrients in Food.” Baltimore: Lippincott Williams & Wilkins, 2000.
Hawkins RA. “The blood-brain barrier and glutamate.” Am J Clin Nutr. 2009 Sep; 90(3): 867S–874S.
Kurihara K. “Glutamate: from discovery as a food flavor to role as a basic taste (umami).” Am J Clin Nutr. 2009 Sep;90(3):719S-722S.
Monro JA, et al. “The risk of lead contamination in bone broth diets.” Med Hypotheses. 2013 Apr;80(4):389-90.
Nakamura E, et al. “Physiological roles of dietary free glutamate in gastrointestinal functions.” Biol Pharm Bull. 2008 Oct;31(10):1841-3.
Pickering MV & Newton P. “Amino acid hydrolysis: Old problems, new solutions.” LC/GC.1990;8(10):778-81.
Pitt D, et al. “Glutamate excitotoxicity in a model of multiple sclerosis.” Nat Med. 2000 Jan;6(1):67-70.
Shinohe A, et al. “Increased serum levels of glutamate in adult patients with autism.” Prog Neuropsychopharmacol Biol Psychiatry. 2006 Dec 30;30(8):1472-7.
Schuette K. “Stock vs. Broth: Are You Confused?” Biodynamic Wellness.
Yang WH, et al. “The monosodium glutamate symptom complex: assessment in a double-blind, placebo-controlled, randomized study.” J Allergy Clin Immunol. 1997 Jun;99(6 Pt 1):757-62.
Zumwalt RW, et al. “Acid hydrolysis of proteins for chromatographic analysis of amino acids.” J Assoc Off Anal Chem. 1987 Jan-Feb;70(1):147-51.